|
Tamarix spp.
|
|
|
|
Scientific name
|
Tamarix spp.
|
|
Additional name information:
|
T. ramosissima Ledeb.; T. chinensis; T. gallica; T. parviflora
|
|
Closely related California natives
|
0
|
|
Closely related California non-natives:
|
5
|
|
Listed
|
CalEPPC List A-1,CDFA nl
|
|
By:
|
Jeffrey Lovich
|
|
Distribution
|
See individual species
åÊ
|
|
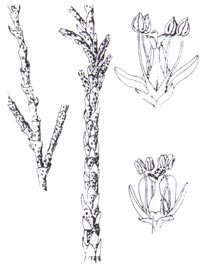
HOW DO I RECOGNIZE IT?
Distinctive features:
|
Four invasive Tamarix species have been
identified in California: T. ramosissima, T. chinensis, T.
gallica, and T. parviflora. All four are many-branched shrubs or
trees less than twenty-six feet tall with small scale-like leaves, from which
comes the name saltcedar. Leaves have salt glands, and salt crystals can often
be seen on leaves. Small white to deep pink flowers are densely arranged on
racemes. The bark is reddish brown with smooth stems less than an inch in
diameter.
åÊ
|
|
Description:
|
|
Tamaricaceae. Tamarix ramosissima is the species described
here. Other invasive Tamarix species are similar, differing slightly in
floral and leaf morphology. Stems: height
|
|
ovate, obtuse to acute, 5 petals, 0.04-0.08 in (1-2 mm) long, elliptic to
oblanceolate, nectar globes wider than long, stamens with alternate disk lobes,
calyx and corolla pentamerous. Seeds: hairy-tufted, 0.02in (
Over 50 species of Tamarix were recognized by Baum (1978), and 5
are reported from California, including T. aphylla, T.
chinensis, T. parviflora, and T. gallica (DiTomaso 1996).
T. aphylla is not an invasive pest under most circumstances. T.
ramosissima may be synonymous with T. chinensis and is sometimes
incorrectly referred to as T. pentandra (Baum 1978).
|
|
WHERE WOULD I FIND IT?
|
Saltcedar is widely distributed throughout
the Mojave and Colorado deserts, OwenÛªs Valley, the Central and South coasts,
and the San Joaquin Valley. It occurs in parts of the San Francisco Bay Area and
the Sacramento Valley, particularly Yolo and Solano counties. French tamarisk
(T. gallica) occurs in the Central Valley, Bay Area, and Central and
South coasts. Smallflower tamarisk (T. parviflora) has a similar range,
but also occurs in Inyo County. Saltcedar is abundant where surface or
subsurface water is available for most of the year, including stream banks, lake
and pond margins, springs, canals, ditches, and some washes. Disturbed sites,
including burned areas, are particularly favorable for saltcedar establishment.
It survives, and even thrives, on saline soils where most native, woody,
riparian plants cannot.
åÊ
|
|
WHERE DID IT COME FROM AND HOW IS IT SPREAD?
|
Tamarix ramosissima is found
throughout much of central Asia, from the Near East around the Caspian Sea,
through western China to North Korea (Baum 1978). Although saltcedar may have
been introduced into North America by the Spaniards, it did not gain recognition
in the western United States until the 1800s (Robinson 1965). It was planted
widely for erosion control, as a windbreak, for shade, and as an ornamental. It
spreads by seed and vegetative growth. Individual plants can produce 500,000
tiny seeds per year (DiTomaso 1996), which are easily dispersed long distances
by wind and water. The roots also sprout adventitiously (Kerpez and Smith 1987,
Lovich et al. 1994).
åÊ
|
|
WHAT PROBLEMS DOES IT CAUSE?
|
There is debate as to whether saltcedar is a consequence
(Anderson 1996) or a cause (Lovich and de Gouvenain 1998) of environmental
changes associated with its presence and proliferation. Regardless, the presence
of saltcedar is associated with dramatic changes in geomorphology, groundwater
availability, soil chemistry, fire frequency, plant community composition, and
native wildlife diversity. Geomorphological impacts include trapping and
stabilizing alluvial sediments, which results in narrowing of stream channels
and more frequent flooding (Graf 1978). Saltcedar has been blamed for lowering
water tables because of its high evapotranspiration rate, and, on a regional
scale, dense saltcedar groves use far more water than native riparian plant
associations (Sala et al. 1996).
Soil salinities increase as a result of inputs of salt from
glands on saltcedar leaves. The dome-shaped glands consist of at least two cells
embedded in the epidermal pits (Decker 1961). Increased salinity inhibits growth
and germination of native riparian species (Anderson 1996). Leaf litter from
drought-deciduous saltcedar increases the frequency of fire. Saltcedar is
capable of resprouting vigorously following fire and, coupled with changes in
soil salinity, ultimately dominates riparian plant communities (Busch 1995).
Although saltcedar provides habitat and nest sites for some
wildlife (e.g., white-winged dove, Zenaida asiatica), most authors have
concluded that it has little value to most native amphibians, reptiles, birds,
and mammals (Lovich and de Gouvenain 1998).
åÊ
|
|
HOW DOES IT GROW AND REPRODUCE?
|
Saltcedar can reproduce both vegetatively
and by seed. Plants can regenerate from cuttings that fall on moist soil. Plants
can flower by the end of the first year of growth (DiTomaso 1996). Studies in
Arizona demonstrated that dense saltcedar stands can generate 100 seeds per
square inch. Seed production occurs over a 5.5-month period, with one major and
one minor peak (Warren and Turner 1975). The minute seeds of the closely related
(Baum 1978) Tamarix gallica are about 0.007 inch (0.17 mm) in diameter
and about 0.018 inch (0.45 mm) long. Small hairs on the apex of the seed coat
facilitate dispersal by wind. Germination can occur within twenty-four hours in
warm, moist soil (Merkel and Hopkins 1957).
|
|
(click on photos to view larger image)
|
Following germination and establishment, the primary root grows
with little branching until it reaches the water table, at which point secondary
root branching is profuse (Brotherson and Winkel 1986). Under favorable
conditions, salt-cedar shoots reportedly grow to heights of 3-4 meters in one
growing season (DiTomaso 1996). Brotherson et al. (1984) examined the
relationship between stem diameter and age of saltcedar plants. Assuming that
observed growth rings of stems are annual, saltcedars in Utah require 7.68 years
for a 0.39 inch (1 cm) increase in stem diameter and 2.36 years in Arizona.
Germination of saltcedar seeds is not greatly affected by increased salinity
under experimental conditions (Shafroth et al. 1995). Saltcedar can form dense
thickets.
åÊ
|
|
HOW CAN I GET RID OF IT?
|
Like most invasive species, saltcedar is
easily spread but difficult to eliminate. Early detection and control are
critical, as saltcedar achieves dominance rapidly under favorable conditions.
Efforts should be made to prevent site disturbances that contribute to its
success (fire, increased soil salinity, ground disturbance, etc.). Monitoring is
essential following any control effort, as some saltcedar is capable of
resprouting following treatment. In addition, seedlings will continue to
establish as long as saltcedar infestations persist upwind or upstream of the
target area.
åÊ
|
|
Physical control:
|
Manual/mechanical methods: Saltcedar is difficult to kill with
mechanical methods, as it is able to resprout vigorously following cutting or
burning. Root plowing and cutting are effective ways of clearing heavy
infestations initially, but these methods are successful only when combined with
follow-up treatment with herbicide. Seedlings and small plants can be uprooted
by hand.
Prescribed burning: Fire does not kill saltcedar roots, and
plants return quickly after fire if untreated by other methods. Fire is valuable
primarily for thinning heavy infestations prior to follow-up application of
herbicide. The consequences of fire for native plants and soil chemistry must be
recognized.
Flooding: Flooding thickets for one to two years can kill most
saltcedar plants in a thicket.
åÊ
|
|
Biological control:
|
Insects and fungi: The USDA is currently
using an international team of researchers to test thirteen species of natural
enemies to control saltcedar. Of these, two have been recommended for field
release in the United States, including a mealybug (Trabutina
mannipara) from Israel and a leaf beetle (Diorhabda elongata) from
China. Two other species are being tested in quarantine, including a psyllid
(Colposcenia aliena) and a gelechiid leaf tier (Ornativalva
grisea) from China. A gall midge (Psectorsema) from France has
been approved for quarantine testing. Overseas testing has been completed for a
foliage-feeding weevil (Coniatus tamarisci) from France, and
for a pterophorid moth (Agdistis tamaricis), and a foliage-feeding
weevil (Cryptocephalus sinaita subsp. moricei) from Israel
(DeLoach 1997).
Grazing: Cattle have been shown to graze significant amounts of
sprout growth (Gary 1960).
åÊ
|
|
Chemical control:
|
Heavy infestations may require stand thinning through controlled
burns or mechanical removal with heavy equipment prior to treatment with
herbicides. Six herbicides are commonly used to combat saltcedar, including;
imazapyr, triclopyr, and glyphosate (Jackson 1996).
Several proven methods exist for removing tamarisk. Perhaps the
best method is to apply an imazapyr marketed as Arsenalå¨ to the foliage. This
technique is especially effective when a tank mix is used with a glyphosate
herbicide such as Rodeoå¨ or RoundupProå¨. The most frequently used method in
California is to cut the shrub off near the ground and apply triclopyr, either
as Garlon 4å¨ or Garlon 3Aå¨. This technique usually results in better than a 90
percent kill rate. Triclopyr (as Pathfinder IIå¨) can even be applied directly to
the basal bark of stems less than about four inches in diameter without cutting
the stem (the bark must be wetted completely around the base of each stem).
Garlon 4å¨or Pathfinder IIå¨ have no timing restrictions, but
Garlon 3Aå¨ should be applied during the growing season. Resprouts can be treated
with foliar applications of herbicide. Foliar applications of glyphosate or
imazapyr achieve best results when applied in late spring to early fall during
good growing conditions. Triclopyr can be diluted with diesel or natural oils, a
dilution of 3 parts water to 1 part of Garlon 4å¨ has proven effective (Barrows
1993, Lovich et al. 1994). Application rates for these herbicides are reviewed
in Jackson (1996). Only Rodeoå¨ has an aquatic registration, making it a legal
choice for application over or around water.
åÊ
|