|
Myriophyllum aquaticum
|
|
|
|
Scientific name
|
Myriophyllum aquaticum
|
|
Additional name information:
|
(Vell. conc.) Verde
|
|
Common name
|
parrotÛªs feather, parrot feather watermilfoil, Brazilian water milfoil
|
|
Synonymous scientific names
|
Myriophyllum brasiliense, M. proserpinacoides, Enydria aquatica
|
|
Closely related California natives
|
3
|
|
Closely related California non-natives:
|
1
|
|
Listed
|
CalEPPC List B,CDFA nl
|
|
By:
|
Kris Godfrey
|
|
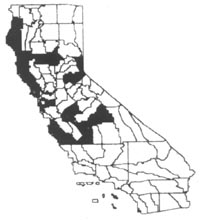
Distribution
|
|
HOW DO I RECOGNIZE IT?
Distinctive features:
|
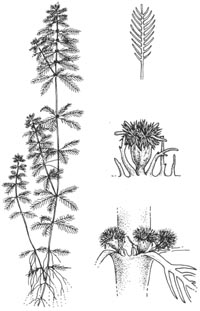
ParrotÛªs feather (Myriophyllum
aquaticum) is a stout aquatic perennial that forms dense mats of
intertwined brownish stems (rhizomes) in water. These stems grow to six and a
half feet in length and resemble bright green bottlebrushes emerging from the
water. The bottlebrush appearance results from the fact that the leaves appear
in whorls of four to six at each node and each leaf is feather-like, the blade
divided into twenty-four to thirty-six thread-like segments. Upon close
inspection the leaves look gray-green. ParrotÛªs feather also has leaves below
the water surface, appearing reddish, feathery, and limp. Unlike other milfoils
(Myriophyllum spp.), parrotÛªs feather stems may grow as much as eight
inches above the water surface (Orchard 1981, Wester-dahl and Getsinger
1988).
åÊ
|
|
Description:
|
| Haloragaceae. ParrotÛªs feather is a perennial, submersed/immersed aquatic plant. Stems: long and unbranched, rooting freely from lower nodes. Leaves: in whorls and slightly dimorphic. Submerged leaves in whorls of 4-6, oblong in appearance, 1.4-1.6 in (3.5-4.0 cm) long, 0.3-0.5 in (0.8-1.2 cm) wide; pectinate with 25-30 linear pinnae to 0.27 in (0.7 cm) long. Lower leaves usually deteriorate rapidly. Emergent leaves glaucous, in whorls of 4-6, oblong, 1-1.4 in (2.5-3.5 cm) long, 0.27-0.31 in (0.7-0.8 cm) wide; pectinate with 18-36 linear pinnae in the upper 80 percent of leaf. Lower 2-2.75 in (5-7 cm) of leaf rachis naked. |
|
Inflorescence: indeterminate spike with
flowers singly in axils of upper emergent leaves. Flowers: only female flowers
known from parrotÛªs feather plants in the United States. Female flowers on
pedicel 0.008-0.02 in (0.2-0.4 mm) long; 4 white sepals, 0.02 in (0.5 mm) long,
0.012 in. (0.3 mm) wide, denticulate with one to several small teeth on each
margin; no petals or stamens, 4 clavate styles 0.004-0.008 in (0.1-0.2 mm) long,
stigmas white and densely fimbriate; ovary pyriform, 0.02-0.03 in (0.6-0.7 mm)
long, 0.02 in. (0.6 mm) wide; 4-ribbed longitudinally between sepals. Fruit:
none in United States (Orchard 1981).
åÊ
|
|
WHERE WOULD I FIND IT?
|
Both parrotÛªs feather and spike
watermilfoil can be found in freshwater lakes, ponds, and canals with
slow-moving waters in northern and central California (Anderson 1990). ParrotÛªs
feather can be found throughout much of the United States from New England to
Florida and westward to California and Washington. Typically, it is found rooted
at depths to 6.5 feet (2 m), but emergent stems may elongate and spread over
deeper waters or to pond edges.
åÊ
|
|
WHERE DID IT COME FROM AND HOW IS IT SPREAD?
|
ParrotÛªs feather is native to South America
and was introduced into the United States in the late 1800s for use in aquaria
and water gardens (Kane et al. 1991). It was first collected in the United
States near Washington D.C. in 1890. It was reported from South Africa in 1918
or 1919, Japan in 1920, New Zealand in 1929, Australia in the 1960s, and England
in the 1970s. A population was reported in western Washington in 1944
(Washington Water Quality Program 1998).
ParrotÛªs feather is capable of sexual reproduction in its native
range, but the spread of parrotÛªs feather in the United States results solely
from vegetative reproduction. The stems of parrotÛªs feather are brittle and
fragment easily. These fragments settle in sediments and produce new plants
(Orchard 1981, Kane et al. 1991). Fragments can be spread by boats, trailers,
and by dumping aquarium plants in waterways. They can also be spread by
waterfowl and other wildlife, as well as by moving water.
åÊ
|
|
WHAT PROBLEMS DOES IT CAUSE?
|
ParrotÛªs feather may compete with native
aquatic plants, eliminating them or reducing their numbers in infested sites. It
forms dense mats that can entirely cover the surface of the water in shallow
lakes and other waterways. These mats clog waterways, making them unusable for
navigation or recreation and causing flooding out of the channel. It can block
irrigation pumps and water intakes, and it provides optimal habitat for
mosquitoes (Orr and Resh 1989, Systma and Anderson 1990; Parsons 1992). In
California this species is becoming an increasing problem in irrigation and
drainage canals. A 1985 survey of irrigation, mosquito abatement, flood control,
and reclamation agencies in California indicated that parrotÛªs feather infested
nearly 600 miles of waterways and over 500 surface acres (Washington Water
Quality Program 1998).
While parrotÛªs feather may provide cover for some aquatic
organisms, it can significantly alter the physical and chemical characteristics
of lakes and streams. Infestations can alter aquatic ecosystems by shading out
algae in the water column that serve as the basis of the aquatic food web. It
also alters habitats for aquatic organisms, waterfowl, and other
wildlife.
åÊ
|
|
HOW DOES IT GROW AND REPRODUCE?
|
Reproduction of
parrotÛªs feather in the United States is believed to be entirely by vegetative
means, resulting from stem fragmentation and/or regrowth from sections of
rhizomes (underground stems) (Jacot Guillarmod 1979, Kane et al. 1991). Even in South America, virtually all parrotÛªs feather plants are female. Male plants are unknown outside South America, so no seeds are produced in North American populations.
With its tough rhizomes, parrotÛªs feather can be transported long distances on boat trailers.
Any rhizome or stem sections with at least one node, even
as small as 0.2 inch (5mm) long, can root and establish new plants.
Rhizomes stored under moist conditions in a refrigerator survived for one year. Once
rooted, these new plants produce rhizomes that spread through sediments and stems that grow
until they reach the water surface (Orchard 1981). The result is a dense, tangled
mass of parrotÛªs feather in the water column.
åÊGrowth is most
rapid from March until September. In spring shoots begin to grow rapidly from
overwintering rhizomes as water temperature increases. Rhizomes function as a support structure for adventitious roots
and provide buoyancy for emergent growth in summer. Emergent stems and leaves
extend from a few inches to over one foot above the water surface. Underwater
leaves tend to senesce as the season advances. Plants usually flower in spring,
but some plants may also flower in fall. The inconspicuous flowers form where
emergent leaves attach to the stem.
|
In fall plants typically die back to the rhizomes. In some areas, parrotÛªs feather may maintain considerable winter biomass. Because the plant lacks tubers, turions, and winterbuds, rhizomes serve all those functions. ParrotÛªs feather does not store phosphorus or carbon in its rhizomes, and this may explain its failure to invade areas with severe winters.
|
(click on photos to view larger image)
|
|
|
HOW CAN I GET RID OF IT?
|
ParrotÛªs feather is difficult to remove
from an aquatic system, so it is best to prevent it from establishing in the
first place. The public must be made aware of the problems caused by parrotÛªs
feather and how it can be spread by dumping unwanted plants from water gardens
or aquaria or by boats, trailers, and fishing equipment that are not cleaned
before being moved to a new waterway. If parrotÛªs feather becomes established,
only chemical and mechanical control methods are available.
åÊ
|
|
Physical control:
|
Mechanical methods: ParrotÛªs feather can be
removed by mechanical harvesters. In Washington, workers use a dragline to
remove parrotÛªs feather plants. A truck-mounted crane with a special attachment
plucks weeds out of the ditch. The dragline operation is conducted annually from
August to December, with control generally lasting for one growing season
(Washington Water Quality Program 1998). Care must be taken to ensure removal of
all plant parts during harvest, since even tiny stem or rhizome fragments can
root and establish new plants. Because of this, mechanical harvesting often
results in the spread of parrotÛªs feather rather than its elimination or
suppression.
åÊ
|
|
Biological control:
|
ParrotÛªs feather has a high tannin content,
so most grazers, including grass carp (Ctenopharyngodon idella), find it
unpalatable. Grass carp also prefer soft plants, such as Elodea canadensis, and
the tough, woody parrotÛªs feather stems are avoided. USDA approved biological
control agents are not currently available. Potential agents do exist, but they
have yet be tested for host specificity. A complex of insects feed on parrotÛªs
feather in its native habitat. Lysathia flavipes, a flea beetle found on
parrotÛªs feather in Argentina, causes moderate damage under field conditions.
Also found in Argentina is a weevil, Listronotus marginicollis, that apparently
feeds only on parrotÛªs feather in its native range. Other insects have been
found on parrotÛªs feather in Florida. Lysathia ludoviciana, a flea beetle native
to the southern United States and the Caribbean, uses parrotÛªs feather as a host
plant for larvae under laboratory conditions. However, the flea beetle is not
often found on parrotÛªs feather in the field. Two members of the Tortricidae
family, Argyrotaenia ivana and Choristoneura parallela, have also been found on
parrotÛªs feather in Florida, but their effect on the plant is unknown. In
addition, larvae of the caterpillar, Parapoynx allionealis, mine parrotÛªs
feather leaves, but the impact of these larvae is unknown.
Fungal control options exist as well. An isolate of Pythium
carolinianum collected in California has shown some promise as a potential
biocontrol agent. ParrotÛªs feather stems experimentally inoculated with this
fungus produced significantly less growth than control plants (Washington Water
Quality Program 1998).
åÊ
|
|
Chemical control:
|
The underwater and above-water foliage of
parrotÛªs feather make herbicides difficult to deliver effectively. Emergent
stems and leaves have a thick, waxy cuticle that inhibits herbicide uptake, and
a wetting agent is required to penetrate it. Often the weight of the spray will
cause emergent vegetation to collapse into the water, where the herbicide is
washed off before it can be translocated throughout the plant. The most recent
version of an herbicide label will give recommended rates and information about
whether the compound is registered for use in specific situations. Herbicide use
is more highly regulated in aquatic systems than in terrestrial systems.
Westerdahl and Getsinger (1988) report excellent control of
parrotÛªs feather with 2,4-D, diquat, diquat and complexed copper, endothall
dipotassium salt, fluridone, and endothall and complexed copper. Diquat is used
on emergent parrotÛªs feather, as well as in the water to kill rhizomes. Copper
complexes are used only on submersed plants. Diquat is not legal for use in
aquatic systems in California. Fair control was obtained with acrolein and
glyphosate. Acrolein is used only in non-fisheries water, and glyphosate,
formulated as Rodeo, is used only on emergent parrotÛªs feather. The Monsanto
Company suggested that applying a 1.75 percent solution of Rodeoå¨ with
surfactant to the plants in summer or fall when water levels are low would give
about 95 percent control. Control of parrotÛªs feather may be achieved with
low-volatility ester of 2,4-D at 4.4-8.9 kg/ha, sprayed onto emergent foliage.
The granular formulation of 2,4-D was needed to control parrotÛªs feather for
periods greater than twelve months. It is more effective when applied to young,
actively growing plants (Washington Water Quality Program 1998).
In practice, weed control efforts report little success with
herbicides to control parrotÛªs feather. Glyphosate causes emergent vegetation to
turn black, but within two weeks the plants have recovered. An experimental fall
application of triclopyr also proved ineffective (Washington Water Quality
Program 1998).
åÊ
|