|
Senecio jacobaea
|
|
|
|
Scientific name
|
Senecio jacobaea
|
|
Additional name information:
|
L.
|
|
Common name
|
tansy ragwort, stinking willie
|
|
Synonymous scientific names
|
none known
|
|
Closely related California natives
|
36
|
|
Closely related California non-natives:
|
5
|
|
Listed
|
CalEPPC List B,CDFA B
|
|
By:
|
Steven A. Harris
|
|
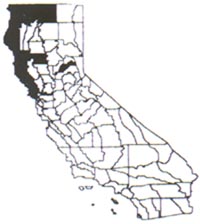
Distribution
|
|
|
HOW DO I RECOGNIZE IT?
Distinctive features:
|
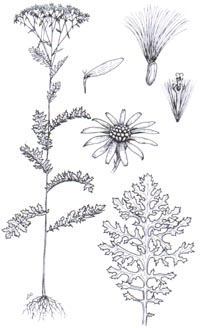
Tansy ragwort (Senecio jacobaea)
is a perennial herb in the dandelion family that sometimes reaches more than
four feet in height. Numerous inch-wide, daisy-like yellow flowerheads with
golden or light brown centers form at the tip of each branch from mid-summer to
fall. The plant has a basal rosette of leaves, and the upper parts are branched.
Leaves are deeply pinnately dissected into irregular segments, giving the plant
a ragged appearance. Leaves or segments are wider than long. Ray flowers
distinguish this plant from common tansy (Tanacetum
vulgare).
åÊ
|
|
Description:
|
|
Compositae. Stems: from taprooted caudex, branched above,
4-50 in (10-120 cm). Leaves: evenly spaced, reduced upward; 2-8+ in (5-20+
cm), ovate, deeply 1-2-pinnately dissected; lower leaves soon deciduous.
Inflorescence: heads radiate, 20-60; 13 phyllaries, 0.15-0.2 in (3-5 mm),
tips green or black. Flowers: 13 ray flowers; ligules about 0.3-0.5 in
(8-12 mm); 40 or more disk flowers. Fruit: glabrous or only edges of fruit
hairy.
åÊ
|
|
|
|
WHERE WOULD I FIND IT?
|
In California tansy ragwort is found on the
North Coast and into the Klamath and Cascade ranges, also occurring in the
Sacramento Valley and San Francisco Bay region. It is commonly found in
pastures, on roadsides, and in disturbed places. It grows best in light, well
drained soils, but can become established in heavier soils, particularly soils
broken up by trampling or frequent cultivation.
åÊ
|
|
WHERE DID IT COME FROM AND HOW IS IT SPREAD?
|
Tansy ragwort is endemic to parts of western Europe and western
Asia, where it is a weed of minor importance on roadsides and grasslands. It is
considered by some authors to be native to the dunes of Holland (van der Meijden
1971). From Europe it has been introduced into Argentina, New Zealand,
Australia, Canada, and the western United States (Schmidl 1972a).
Tansy ragwort seeds, like those of other members of the
Asteraceae, are assumed to be primarily wind-dispersed (van der Meijden 1971).
Plants are commonly found along roadsides in otherwise uninfested areas (Holden
1989).
Animal transport of ragwort achenes seems likely. The pappus and
bristle hairs of disk achenes would allow attachment to fur and feathers. Plants
have been commonly observed along deer and elk trails through otherwise
uninfested regions (Holden 1989).
Tansy ragwort has been transported in infested hay. This mode of
dispersal was postulated to explain several isolated infestations observed in
eastern Oregon when hunting parties carried hay into previously uninfected areas
(Cox and McEvoy 1983).
The plant can also spread via regeneration of root fragments
contained in mud or soil adhering to vehicles.
åÊ
|
|
WHAT PROBLEMS DOES IT CAUSE?
|
Tansy ragwort is highly toxic to livestock.
It contains pyrrolizidine alkaloids that cause liver damage in horses and cattle
(Cheeke 1979). Cattle and horses are affected seriously; goats may suffer
poisoning; sheep are generally not poisoned by this plant. Tansy ragwort easily
outcompetes native and naturalized grasses and forbs (Harper 1958). It is
estimated to occur on three million acres in western Oregon, where two out of
every five acres of pasture are infested. More than 112,000 acres of pasture in
western Washington contain tansy ragwort. It has been reported in Idaho and is
there considered a serious potential weed problem that has not yet reached a
level of economic importance. Most ragwort is on forested and clearcut lands
(Bedell et al. 1995).
åÊ
|
|
HOW DOES IT GROW AND REPRODUCE?
|
Tansy ragwort is a biennial species. The
first-year plant is a rosette of basal leaves that are raggedly lobed and to
nine inches (22.5 cm) long. In the second year the first-year leaves die back
and the plant develops tall, leafy flowering stems in summer. It is ordinarily
about three and one-third feet (1 m) in height, but plants to ten feet (3 m) are
not uncommon, with many branches near the top (Holden 1989).
Tansy ragwort is capable of regeneration from
pieces of rootstock. Roots without any portion of the root crown are able to
produce new plants, and even severed roots of cotyledons can grow new shoots
(Holden 1989). Poole and Cairns (1940) found that first-year plants, or
rosettes, buried five inches (12.5 cm) in the soil sprouted three months later,
even in heavy soil.
In addition to vegetative propagation,
ragwort also reproduces by seed. Two studies at different sites recorded 4,760
to 174,230 seeds per plant (Camoron 1935) and 6,480 to 137,500 seeds per plant
(Poole and Cairns 1940).
Ragwort inflorescences contain two types of
flowers, an outer row of radiate (ray) female flowers and many central tubular
(disk) perfect flowers. Each flower type produces distinctive single-seeded
fruits (achenes) that have different dispersal mechanisms, germination
requirements, and germination rates (Harper 1965, Burtt 1977). Seeds are tipped
by hair-like plumes that carry seeds in the wind for long distances. Although
both fruit types are primarily dispersed by wind, disk achenes are dispersed
earlier and farther than ray achenes (Green 1937). Poole and Cairns (1940) found
that 60 percent of the total seed shed landed within fourteen feet (4.6 m) of
the base of the plant; an additional 39 percent landed between fourteen and
twenty feet (4.6 and 9 m) from the plant.
(click on photos to view larger
image)
|
Tansy ragwort seeds remain viable in
the soil for several years. In this way the species can wait for favorable
conditions to occur. McEvoy (unpubl. data) found 0.75 viable ragwort
seeds/cc in the upper 1.2 inches (3 cm) of soil underneath a pasture
heavily infested with ragwort. Viable seeds were encountered in samples
taken as deep as ten inches (25 cm) below the surface.
On the Oregon coast, tansy ragwort
seeds mature in late summer and early fall. Seeds reach maximum
germination potential before dispersal; they do not possess an
innate
|
|
dormancy
(van der Meijden and van der Waals-kooi 1979). There are two peaks of
germination: fall and spring. However, some germination occurs year round
(Harper and Wood 1957).
Soil moisture, soil surface humidity, and
light are important factors in ragwort germination. Van der Meijden and van der
Waals-kooi (1979) found germination of ragwort achenes to be greater at soil
moisture levels between 15 and 29 percent and a relative humidity at the soil
surface of 100 percent.
Ragwort needs an opening through some form of
disturbance to become established (van der Meijden and van der Waals-kooi 1979).
Several disturbance agents have been cited, including moles and gophers, ants,
rabbits, livestock, and humans (Harper and Wood 1957).
åÊ
|
|
HOW CAN I GET RID OF IT?
|
åÊ
|
|
Physical control:
|
Mechanical methods: Deep plowing has been
unsuccessful in controlling tansy ragwort (Holden 1989). This technique severs
roots and distributes them over a wide area. Plowing also unearths buried seeds
and can contribute to a more severe infestation (Holden 1989).
Mowing has been widely used in attempts to prevent the spread of
ragwort, but has provided only cosmetic control. Repeated mowing could deplete
reserves and eventually exhaust the population. Fields would have to be mowed
every six weeks during spring and summer months and be accompanied by moisture
stress (Cox and McEvoy 1983). Single mowings during flowering might intensify
local infestations. Given that ragwort seeds ripen over a range of time and that
severed capitula are able to produce seed, mowing might increase local seedling
density while freeing the parent plant from reproductive burdens and engendering
basal sprouting (Holden 1989).
Hand pulling has been the most common technique used on small
pastures in the early stages of infestation. Soil moisture is critical, as drier
soils allow root breakage and pulling in wet soils removes large soil clumps.
Partially opened flowers continue to set viable seed if sufficient moisture is
available in the cut plant. Therefore, plants must be removed from the treated
site and buried or burned (Holden 1989).
Clipping or otherwise deflowering plants eliminates seed set and
prevents further spread if flowerheads are collected and burned (Holden 1989).
The original plant, however, continues to sprout, grow, and reflower in
subsequent seasons.
Prescribed burning: Burning is traditionally used in
agricultural croplands as a preemergence weed treatment. In ragwort control,
Poole and Cairns (1940) experimented with a flame thrower, but their results
were inconclusive and have not been reproduced by others. Mastroguiseppe et al.
(1982) conducted several control burns at an infested site in Redwood National
Park, California, but the results were inconclusive.
åÊ
|
|
Biological control:
|
Insects and fungi: Biological control has proven to be effective
for long-term control of extensive infestations of tansy ragwort. Several USDA
certified biocontrol agents have been released for control of this weed. The
cinnabar moth, Tyria jacobaeae, is a day-flying moth
indigenous to Europe and Asia. Cinnabar larvae feed primarily on the flower
buds, but readily consume leaves and stems as well. Unfortunately, the cinnabar
moth is subject to disease, predation, and parasitism.
Tansy seedfly (Pegohylemia seneciella) is a small
muscid fly common in tansy flowers in France and Italy. The seedfly consumes the
seeds of tansy, but does not kill or inhibit growth of the host
plant.
Tansy flea beetle, Longitarsus jacobaeae, is another
host-specific pest of tansy that is indigenous to Mediterranean Europe. Flea
beetle larvae feed throughout the root crown and sometimes feed externally on
lateral roots. Larvae bore into the stem and leaf petioles for two to five
inches (5-10 cm), causing wilt and death of the plant. Plants seldom recover
from a heavy infestation of the beetle. Adult flea beetles feed on the leaves,
causing a characteristic ÛÏshot-holeÛ pattern.
Holden (1989) obtained dramatic results from introduction of the
flea beetle; percent absolute cover of ragwort declined from a mean of 22.7 in
the fall 1982 to zero in summer 1984. At the beginning of this study (October
1982) ragwort cover in the circular vegetation plots ranged from less than 1
percent to 38 percent. By July 1984, all measured plots showed zero
cover.
Vegetative competition: In New Zealand many hectares of land
were converted to forests by planting commercial trees over infested sites
(Holden 1989).
Grazing: Sheep grazing reduced the density of tansy ragwort, but
the effect appears to be temporary. Ragwort reappeared as soon as the sheep were
removed (Holden 1989).
åÊ
|
|
Chemical control:
|
The first chemical used to combat tansy
ragwort was the soil sterilant, sodium chlorate, a non-selective inorganic
compound. The high cost of sodium chlorate prevented its use as a widespread
control agent. Whitson et al. (1985) recommends dicamba (as Banvelå¨) for ragwort
control at label concentrations. Ragwort plants treated with herbicides have
been found to be more readily eaten by livestock, so grazing must be
discontinued. Cattle may reenter the field only after the plants have completely
dried and been replaced by other forage species (Holden 1989). Often herbicides
have not been effective in killing this plant because the herbicide leached out
of the roots without being transported throughout the plant (Poole and Cairns
1940).
Removal of individual ragwort plants or small populations
requires immediate attention. To maintain control of tansy ragwort, a
combination of control efforts must be diligently applied, and every opportunity
must be taken to eliminate outlying individuals and populations.
åÊ
|