|
Arundo donax
|
|
|
|
Scientific name
|
Arundo donax
|
|
Additional name information:
|
(L.)
|
|
Common name
|
giant reed, giant cane
|
|
Synonymous scientific names
|
none known
|
|
Closely related California natives
|
0
|
|
Closely related California non-natives:
|
0
|
|
Listed
|
CalEPPC List A-1,CDFA noxious
|
|
By:
|
Thomas Dudley
|
|
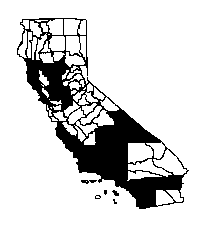
Distribution
|
|
|
HOW DO I RECOGNIZE IT?
Distinctive features:
|
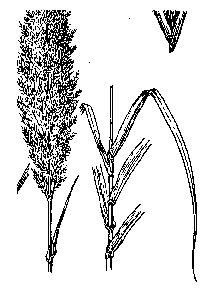
Giant reed (Arundo donax) is a robust perennial grass
nine to thirty feet tall, growing in many-stemmed, cane-like clumps, spreading
from horizontal rootstocks below the soil, and often forming large colonies many
meters across. Individual stems or culms are tough and hollow, divided by
partitions at nodes like bamboo. First-year culms are unbranched, with single or
multiple lateral branches from nodes in the second year. The pale green to
blue-green leaves, which broadly clasp the stem with a heart-shaped base and
taper to the tip, are up to two feet or more in length. Leaves are arranged
alternately throughout the culm, distinctly two-ranked (in a single plane).
Giant reed produces a tall, plume-like flowerhead at the upper tips of stems,
the flowers closely packed in a cream to brown cluster borne from early summer
to early fall. Culms may remain green throughout the year, but often fade with
semi-dormancy during the winter months or in drought. Giant reed can be confused
with cultivated bamboos and corn, and in earlier stages with some large-stature
grasses such as Leymus (ryegrass), and especially with Phragmites
(common reed), which is less than ten feet tall and has panicles less than one
foot long with long hairs between the florets.
|
|
Description:
|
Poaceae. Stems: 30 ft ( internodes, ligule thinly membranous and
fringed with hairs; blade |
|
Inflorescence: as terminal panicle 1-2 ft (30-60 cm) with
branches ascending, silver-cream-brown, the numerous spikelets laterally
compressed; glumes > florets, membranous and 3-5 veined; florets 4-5,
breaking above glumes; lemma 0.3-0.5 in (8-12 mm) and hairy, nerves ending
in slender teeth, the middle forming an inconspicuous awn; palea
|
|
WHERE WOULD I FIND IT?
|
Giant reed occurs in central and southern
California and in Baja California, usually below 1,000 feet (350 m) elevation.
It has invaded central California river valleys in San Luis Obispo and Monterey
counties, the San Francisco Bay Area, and in the Sacramento and San Joaquin
River valleys, and is also increasing in the North Coast region (Dudley and
Collins 1995). Giant reed has been the most serious problem in coastal river
drainages of southern California, especially in the Santa Ana, Santa Margarita,
Santa Clara, Tijuana, and other major and minor watersheds, where it sometimes
occupies entire river channels from bank to bank (Jackson et al. 1994, Bell
1998). Although not currently considered a problem in California deserts, giant
reed survives in regularly watered areas of lower-elevation deserts, but does
not appear to tolerate high-elevation and continental environments where regular
freezing occurs (Sunset 1967).
Giant reed is naturalized and invasive in
many regions, including southern Africa, subtropical United States through
Mexico, the Caribbean islands and South America, Pacific Islands, Australia, and
Southeast Asia (Hafliger and Scholz 1981). In California, the largest colonies
occur in riparian areas and floodplains of medium-sized to large streams, from
wet sites to dry river banks far from permanent water. Giant reed tends to favor
low-gradient (less that 2 percent) riparian areas over steeper and smaller
channels, but scattered colonies are found in moist sites or springs on steeper
slopes.
Populations also occur in the upper estuaries of coastal
streams. It is often found along drainage ditches, where the plant has been used
for bank stabilization, and in other moist sites, including residential areas
where giant reed is used horticulturally. While it is usually associated with
rivers that have been physically disturbed and dammed upstream, giant reed also
can colonize within native stands of cottonwoods, willows, and other riparian
species, even growing in sites shaded by tree canopy. Plants establish primarily
in streamside sites, but expand beyond the margins of riparian vegetation.
Soil preferences are broad, as giant reed is known from coarse sands to
gravelly soil to heavy clays and river sediments. It grows best in well drained
soil with ample moisture, from freshwater to semi-saline soils at margins of
brackish estuaries. In Egypt, Rezk and Edany (1979) found that Arundo
donax tolerates both higher and lower water table levels than Phragmites
australis, which is native to California.
|
|
WHERE DID IT COME FROM AND HOW IS IT SPREAD?
|
Three species of Arundo occur worldwide in tropical to
warm temperate regions. A. donax is often considered indigenous to the
Mediterranean Basin (Hickman 1993) or to warmer regions of the Old World, but
apparently it is an ancient introduction into Europe from the Indian
sub-continent (Bell 1998). In Eurasia it similarly inhabits low-gradient river
courses and may provide useful wildlife habitat in greatly altered river deltas
(Granval et al. 1993, He 1991).
Giant reed was brought to North America
quite early, as it was abundant by 1820 in the Los Angeles River, where it was
harvested for roofing material and fodder. This plant has played an important
role in the development of music, as the cane was the source of the original Pan
pipe or syrinx, and remains the source of reeds for woodwind instruments (Perdue
1958). Commercial plantations exist in California for musical instrument
production, and other commercial possibilities are being explored. Horticultural
propagation is widely conducted, and varieties of Arundo are available and
commonly used in gardens or for erosion control (Sunset 1967). Invasive
populations almost certainly resulted from escapes and displacement of plants
from managed habitats. It spreads vegetatively either by rhizomes or
fragments.
|
|
WHAT PROBLEMS DOES IT CAUSE?
|
Giant reed displaces native plants and associated wildlife
species because of the massive stands it forms (Bell 1994, Gaffney and Cushman
1998). Competition with native species has been shown to result from
monopolization of soil moisture and by shading (Dudley unpubl. data). It clearly
becomes a dominant component of the flora, and was estimated to comprise 68
percent of the riparian vegetation in the Santa Ana River (Douthit 1994). As
giant reed replaces riparian vegetation in semi-arid zones, it reduces habitat
and food supply, particularly insect populations, for several special status
species such as least Bell’s vireo, southwestern willow flycatcher, and
yellow-billed cuckoo (Frandsen and Jackson 1994, Dudley and Collins 1995).
Unlike native riparian plants, giant reed provides little shading to the
in-stream habitat, leading to increased water temperatures and reduced habitat
quality for aquatic wildlife. At risk are protected species such as arroyo toad,
red-legged frog, western pond turtle, Santa Ana sucker, arroyo chub, unarmored
three-spined stickleback, tidewater goby, and southern steelhead trout, among
others (Franklin 1996). In the Sacramento-San Joaquin Delta region Arundo
donax interferes with levee maintenance and wildlife habitat management
(Perrine, pers. comm.).
Giant reed is also suspected of altering
hydrological regimes and reducing groundwater availability by transpiring large
amounts of water from semi-arid aquifers. It alters channel morphology by
retaining sediments and constricting flows, and in some cases may reduce stream
navigability (Lake, pers. comm., TNC 1996).
Dense growth presents fire
hazards, often near urbanized areas, more than doubling the available fuel for
wildfires and promoting post-fire regeneration of even greater quantities of
giant reed (Scott 1994, Gaffney and Cushman 1998). Uprooted plants also pose
clean-up problems when deposited on banks or in downstream estuaries (Douthit
1994) and during floods create hazards when trapped behind bridges and other
structures. Although often planted for erosion control, giant reed can promote
bank erosion because its shallow root system is easily undercut and bank
collapse may follow.
|
|
HOW DOES IT GROW AND REPRODUCE?
|
Plants in North America do not appear to produce viable
seed, and seedlings are not seen in the field. Population expansion here
occurs through vegetative reproduction, either from underground rhizome
extension of a colony or from plant fragments carried downstream,
primarily during floods, to become rooted and form new clones.
Horticultural propagation is routinely done by planting rhizomes, which
readily establish, but stems with no basal material are less likely to
root. Fresh stems form roots at nodes under laboratory conditions, but
survival is poor (Zimmerman and Bunn unpubl. data), and root formation
does occur where an attached culm has fallen over and is in contact with
the substrate.
|
New shoots arise from rhizomes in nearly any
season, but are most common in spring. Growth likewise occurs in all
seasons, but is highly sensitive to temperature and moisture (Perdue
1958). During warm months with ample water culms are reported to attain
growth rates of 2.3 feet (70 cm) per week or about four inches (10 cm) per
day, putting it among the fastest growing terrestrial plants. Biomass
production has been estimated at 8.3 tons dry weight per acre (Perdue
1958). Young stems rapidly achieve the diameter of mature canes, with
subsequent growth involving thickening of the walls (Perdue 1958).
|
(click on photos to view larger image)
|
|
Age of individual culms is certainly more than one year,
and branching seems to represent stem growth in later years, while
rhizomes show indeterminant growth. Branches also form when a stem is cut
or laid over. Dieback is infrequently observed, but culms fade or
partially brown out during winter, apparently becoming dormant under cold
conditions. The outstanding growth trait of this plant is its ability to
survive and grow at almost any time under a wide variety of environmental
conditions.
|
|
|
HOW CAN I GET RID OF IT?
|
Studies of giant reed invasion in California are underway, so more data on its biology and management will be available soon. For further information about monitoring and managing infestations, contact Team Arundo in southern California or Team Arundo del Norte in central and northern parts of the state (see Resources section).
|
|
Physical control:
|
Manual methods: Minor infestations can be
eradicated by manual methods, especially where sensitive native plants and
wildlife may be damaged by other methods. Hand pulling is effective with new
plants less than six feet (2 m) in height, but care must be taken that all
rhizome material is removed. This may be most effective in loose soils and after
rains have made the substrate workable. Plants can be dug up using hand tools
(pick-ax, mattock, and shovel), especially in combination with cutting of stems
near the base with pruning shears, machete, or chainsaw. Stems and roots should
be removed or burned on site to avoid re-rooting, or a chipper can be used to
reduce material, although clogging by the fibrous material makes chipping
difficult (Dale, pers. comm.). For larger infestations on accessible terrain,
heavier tools (rotary brush-cutter, chainsaw, or tractor-mounted mower) may
facilitate biomass reduction, followed by rhizome removal or chemical treatment.
Such methods may be of limited use on complex or sensitive terrain or on slopes
over 30 percent, and may interfere with reestablishment of native plants and
animals.
Mechanical methods: Mechanical eradication is extremely
difficult, even with a backhoe, as rhizomes buried under three to ten feet (1-3
m) of alluvium readily resprout (R. Dale, pers. comm., Else et al. 1996).
Removal of all such material is infeasible, especially where extensive soil
disturbance would be disruptive.
Prescribed burning: In most
circumstances burning of live or chemically treated material should not be
attempted, as it cannot kill the underground rhizomes and probably favors giant
reed regeneration over native riparian species (Gaffney and Cushman 1998).
Burning in place is problematic because of the risks of uncontained fire, the
possibility of damage to beneficial species, and the difficulties of promoting
fire through patchily distributed stands. There may be some cases where burning
of attached material can be done, but only if other means of reducing biomass
cannot be carried out. Cut material is often burned on site, subject to local
fire regulations, because of the difficulty and expense involved in collecting
and removing or chipping all material.
|
|
Biological control:
|
Insects and fungi: No
biological control agents against Arundo donax have been approved by the
USDA, although some invertebrates are known to feed on the grass in
Eurasia/Africa (Tracy and DeLoach 1999). The green bug (Schizaphiz
graminum) has been observed to feed on giant reed in winter (Zuniga et al.
1983). In France Phothedes dulcis caterpillars may feed on it. The insect
Zyginidia guyumi uses giant reed as an important food source in Pakistan
(Ahmed et al. 1977). A moth borer (Diatraea saccharalis) has been
reported to attack it in Barbados. A USDA evaluation of the potential benefits
of biological control against giant reed ranked it as a promising candidate and
suggested several insects and pathogens as possible control agents (Tracy and
DeLoach 1999).
Grazing: Vertebrate grazers such as cattle and
sheep may be useful in controlling giant reed, and Angora goats have been
partially successful in reducing this plant and other brush in southern
California (Daar 1983). Grazers are unlikely to reduce population size
sufficiently to eliminate the risks posed. Likewise, management of native plants
to increase competition with giant reed probably provides insufficient control,
and in fact seems to offer little resistance against the invading
reeds.
|
|
Chemical control:
|
In many, if not all, situations it may be
necessary to use chemical methods to achieve eradication, especially in
combination with mechanical removal. The most common herbicidal treatment
against giant reed is glyphosate, primarily in the form of Rodeoå¨, which is
approved for use in wetlands (Round-Upå¨ can be used away from water). Because
glyphosate is a broad-spectrum herbicide, care should be taken to avoid
application or drift onto desirable vegetation. The standard treatment is a
foliar spray application of 1.5 percent by volume glyphosate with a 0.5 percent
non-ionic surfactant (Monsanto 1992). Most effective application is
post-flowering and pre-dormancy, usually late August to early November when
plants are translocating nutrients into root and rhizomes (TNC 1996). Foliar
uptake and kill may be achieved by spray application during active growth
periods, primarily late spring through early fall (Monsanto 1992). Small patches
can be treated from the ground using backpack or towed sprayers, and major
infestations have been aerially sprayed using helicopters.
Direct
treatment to cut culms can reduce herbicide costs and avoid drift onto desirable
plants, with fair results year-round and best kill in fall, although it appears
to be more successful in shaded sites (Else et al. 1996, Vartanian, pers.
comm.). Concentrated glyphosate solution (50 percent to 75 percent Rodeo, or 27
percent to 40 percent glyphosate is applied to stems, cut within two to four
inches (5-10 cm) of the substrate, by painting with a cloth-covered wand or a
sponge or spraying with a hand mister. It may be helpful to add a dye or food
coloring to the solution to identify treated material. Solution must be applied
immediately following cutting because translocation ceases within minutes of
cutting; a five-minute maximum interval is suggested (TNC 1996).
New
growth is sensitive to herbicides, so a common alternative is to cut or mow a
patch and allow regeneration, returning three weeks to three months later when
plants are three to six feet (1-2 m) tall to treat new growth by foliar spraying
of glyphosate. Promoting regrowth causes nutrients to be drawn from the roots,
potentially reducing the movement of glyphosate to the roots (TNC 1996). With
all methods, follow-up assessment and treatment should be conducted, and some
professional applicators suggest six return spot treatments over six months (Van
Diepen, pers. comm.). Other chemical control methods have been tested, including
paraquat and triclopyr compounds (Arnold and Warren 1966, Horng and Leu 1979,
Franklin 1996), but are not recommended near water.
|